J. Saven & I. J. Dmochowski
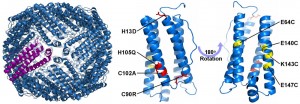
X-ray crystal structure of a designed human H ferritin and a single 4-helix bundle subunit indicating computationally designed mutations to the exterior (red) and interior (yellow) surfaces.
Human H ferritin (HuHF) forms an assembly comprising 24 copies of a four-helix bundle protein. The resulting ~500 kDa protein possessesan 8 nm internal cavity. HuHF was computationally re-designed to facilitate noble metal ion binding, reduction, and nanoparticle formation within the cavity. In addition, computationally determined amino acid substitutions were targeted at four external and four internal surface sites. The engineered proteins retained wild-type stability and structure, as confirmed by X-ray crystallography. A variant with 96 non-native internal cysteines promoted the formation of silver and gold nanoparticles within the protein cavity. This work illustrates how natural proteins and protein nano-contaners may be reengineered so as to confer abiotic metal binding, nanocluster formation, and protein encapsulation.
