Diffusion in Ionomer
Ionomers are copolymers containing a non-polar backbone and a low concentration of fully or partially neutralized acid groups. Ionomers form ion-rich aggregates because of the strong association of ion groups, which inhibits the mobility of ionomers. Despite considerable advances, diffusion in ionomers is not well understood. It is known that diffusion in polymer melts has extensively been studied since the last two decades. The reptation model has provided us the theoretical framework to describe the dynamics of entangled flexible polymers. In 1991, Leibler et al. proposed the sticky reptation model to describe the dynamics of entangled networks made up of linear chains with many temporary cross-links. However, up to now, there is no direct experiment to test this model. In our research, the diffusion of ionomer chains into matrices of another ionomer chains is studied by forward recoil spectrometry. Our ionomer system is based on sulfonated polystyrene, which is neutralized by different cations. The diffusion coefficient is predicted to depend on chain length, acid concentration, and the interaction energy among those ion aggregates.
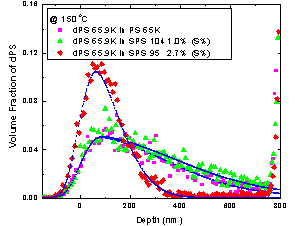
To date, we have studied the diffusion of homopolymer deuterated polystyrene (dPS) in random copolymer, i.e., sulfonated polystyrene (SPS). Diffusion coefficient decreases with increasing the concentration of sulfuric acid group. It is also noticed dPS and SPS become partial immiscible with increasing concentration, until immiscible at relatively high concentration. All these observations are shown in the figure. The slowdown of diffusion of dPS in SPS is explained due to the fact that the monomeric friction coefficient increases with the concentration of sulfuric acid group.
Reference:
(1) Doi, M.; Edwards, S. F.; The Theory of Polymer Dynamics, Oxford, 1986
(2) Leibler, L.; Rubinstein, M.; Colby, R. H. Macromolecules 1991, 24, 4701
(3) Colby, R. H.; Zheng, X.; Rafailovich, M. H.; etc., Phys. Rev. Lett. 1998, 81, 3876