In-situ preparation, characterization and properties of Ag-nanoparticle containing polymer thin films.
In recent years lot of research has undergone
towards the synthesis of nanoparticle polymer composites because of their
potential applications as optical, electronic, magnetic materials. The
properties can be tailored by changing particle size, size distribution, shape
and particles themselves. The key problems in this area involve synthesis,
functionalization of the nanoparticles and dispersion in a polymer matrix.
Here we present an alternative route for the
in-situ production of silver (Ag) nanoparticles in PMMA thin films. The method
used for the in situ synthesis of nanoparticles is known as thermal
decomposition method. Silver precursors such as Ag-HFA have been used in MOCVD
applications. The decomposition temperature of the complex was
found out to be from 160 to 200 °C.
PMMA was added to this
silver-complex containing solution and thin films were cast on silicon
substrate. The films are pre-annealed in vacuum at 110 °C for 24h to
remove the entrapped solvent followed by annealing at 185 °C in for
different times ranging from 30 minutes to 10 days. Annealing causes decomposition of metal-organic complex and in
situ formation of nanoparticles in PMMA matrix.
The formation of the nanoparticles is confirmed
by the TEM. The distribution of silver nanoparticles in the PMMA is analyzed by
Rutherford back Scattering (RBS) showing surface segregation of nanoparticles.
More studies are currently under progress to understand the surface segregation
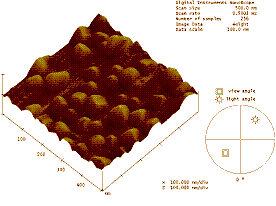
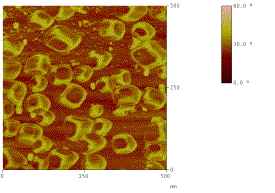
phenomena. Atomic Force Microscopy (AFM) is used to characterize the surface of
the thin films as shown in figure below showing faceted nanoparticles.
UV-Visible spectroscopy on the PMMA/Ag composite films shows plasmon resonance
due to the presence of nanoparticles.
|
|
|
Figure: Topology (left) & phase (right) AFM images showing faceted nanoparticles on the surface of polymer (PMMA)
References:
1). Rubira F. A. , Rancourt J. D., Caplan M. L., Clair A. K., Taylor L. T., Chem.
Mater. (1994), 6, 2351-2358.
2). Southward R. E.,
Thompson D. W., Materials & Design, 22 (2001), 565-576.