M. M. Sheehan, S. E. Chobot, J. L. R. Anderson, G. R. Wiedman, C. C. Moser, D.A. Bonnell, B. M. Discher, and P. Leslie Dutton
Central image represents model of an a-helical bundle which can execute many different functions, in a similar way these functions are accomplished by natural proteins such as myoglobin (top left), NADPH oxidase (bottom); and cytochrome bc1 (top right image).
We have designed and fabricated simple artificial protein scaffolds (we call them maquettes) that can transfer catalytic functions familiar in Nature into materials. Our proteins, built just from four alpha helices, proved to be very simple and robust structural element for variety of functions. To date, we have demonstrated that four-helical maquettes can (1) bind oxygen and CO as it is done by natural oxygen carriers (such as myoglobin, hemoglobin, or neuroglobin) and have high promise to be developed into red blood cell substitutes; (2) generate superoxide in similar fashion white blood cell defend our body against both infectious disease and foreign materials; (3) catalyze chemical reactions seen in Nature, for example quinol-cytochrome c oxidoreductase activity; and (4) transport electrons across membranes in a way cells generate energy. This scaffold represents a new product that can be used as material for clinical, agricultural and sustainable energy devices.
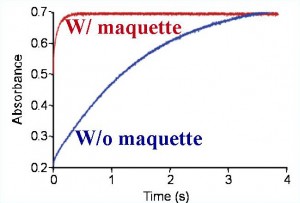
Electron transfer across membrane
Four helix maquettes assembled with cofactors into lipid membranes transfer electrons significantly faster (red line) than cofactors freely diffusing within the membrane (blue line).
Biophysical Journal, V98, 639a, January 2010
Biophysical Journal, V98, 565a, January 2010
