Tobias Baumgart and Ivan J. Dmochowski
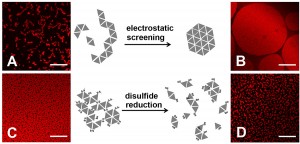
Figure 2. Morphology of protein assembly varied with ionic strength and addition/removal of reducing agent. Assembly of HSA-TR (0.010 mg/mL) was investigated under four conditions:
(A) 10 mM phosphate, 30 mM NaN3, 140 mM NaCl,
(B) 10 mM phosphate, 30 mM NaN3, 500 mM NaCl,
(C) 10 mM phosphate only, and
(D) 10 mM phosphate, 30 mM dithiothreitol.
Scale bar = 20 mm
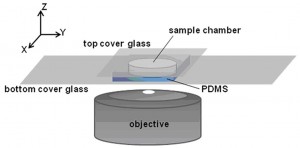
Protein assembly at the air-water interface (AWI) occurs naturally in many biological processes, and provides a method for creating ordered biomaterials. However, the factors that control protein self-assembly at the AWI are generally not well understood. Here, we describe the behavior of a model protein, human serum albumin minimally labeled with Texas Red dye (HSA-TR), using a new confocal microscopy technique (Figure 1). Albumin was observed to form well-ordered, mesoscale monolayer structures at the AWI (Figure 2), which depended on protein concentration, ionic strength, redox state and surfactant. We are investigating thermodynamic and kinetic details of assembly.