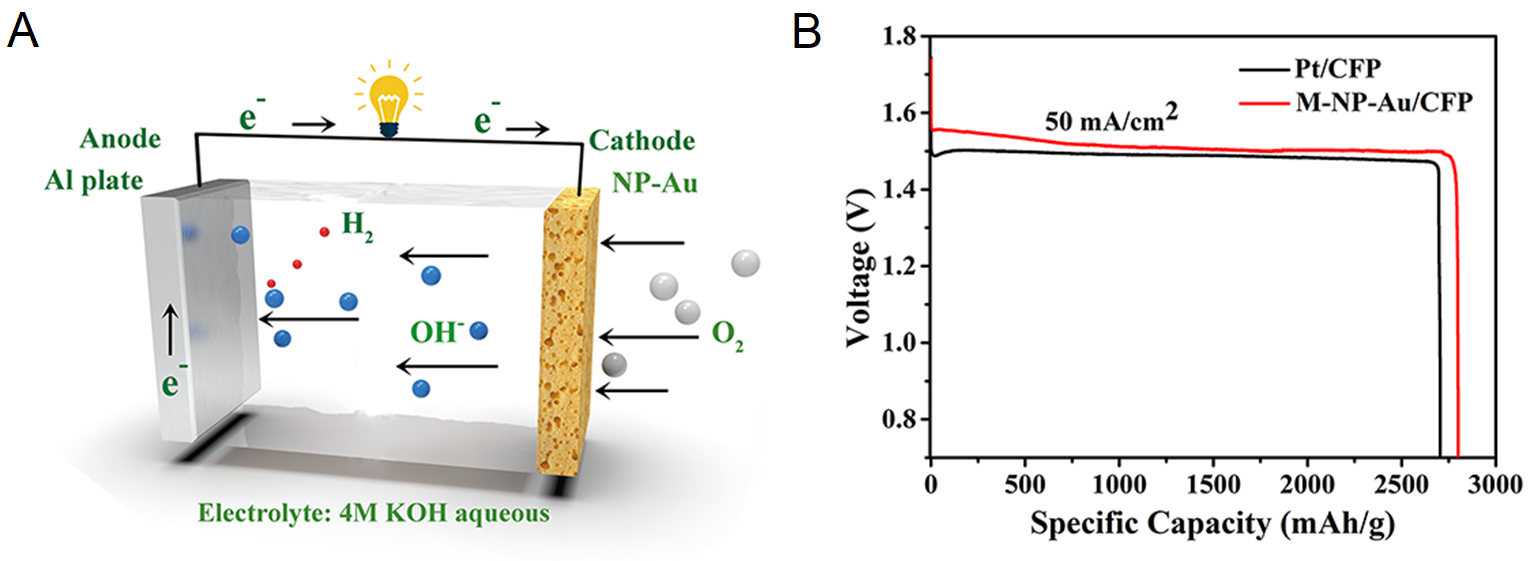
(A) A schematic of an aluminum-air battery, where aluminum is oxidized at the anode (left) and oxygen is reduced at the cathode (right).
(B) Specific capacities of Al-air batteries with our modified nanoporous gold (M-NP-Au/CFP), which outperforms commercial platinum carbon (Pt/CFP) electrodes at 50 mA/cm2 current density.
Intellectual Merit:
Nanoporous (Np) metals have become increasingly important in energy applications due to their excellent material properties. There is, however, a lack in understanding of the mechanisms that control dynamic evolution of Np metal surfaces during synthesis, which limits control of their properties.
The goal of this seed is to develop and validate new synthesis, characterization, and measurement techniques for understanding the process–property–performance of Np gold synthesized by surfactant-modified dealloying. We have shown that dealloying Np gold in a solution with sodium citrate increased the fraction of exposed (100) facets. The modified Np gold electrodes increased the potential, capacity, and power of aluminum-air batteries, so that they had 65% greater energy density than conventionally synthesized Np gold.
Additionally, we (led by Detsi) have investigated the formation of nanoporous gold (NP-Au) in non-oxidizing acids taking advantage of a catalytically driven oxygen reduction reaction (ORR). In general, NP-Au films are commonly made by free corrosion dealloying of gold-silver (Au-Ag) alloys in concentrated nitric acid with molarity higher than 15 M. The use of highly concentrated nitric acid, a strong oxidizing agent, is undesirable because of its very hazardous nature (nitric acid is extremely corrosion, it can rapidly burn upon direct contact, it can cause severe health issues if inhaled). Therefore, producing NP-Au films in non-oxidizing acid is desirable.
Thermodynamically, the spontaneous dissolution of silver in non-oxidizing acids is not favorable because in a non-oxidizing acid, the counter reaction during metal corrosion corresponds to the reduction of hydrogen protons into hydrogen gas, a reaction which generates only 0.0 V versus standard hydrogen electrode (SHE). This 0.0 V is far below the Ag oxidation potential of 0.79 V versus SHE. In our work, we have demonstrated the use of a catalytically driven oxygen reduction reaction (ORR) to induce the dissolution of silver from Au-Ag alloys in diluted hydrochloric acid (2 M) at ambient temperature and pressure. This was achieved by simply replacing the hydrogen proton reduction reactions (which generates at most 0.0 V vs SHE) by the oxygen reaction reaction (which generates at most 1.23 V SHE). The voltage of 1.23 V is high enough to dissolve Ag (at least 0.79 V is required to dissolve Ag). In that way, Ag is selectively removed from Au-Ag alloys in a non-oxidizing acid, resulting in the formation of NP-Au.
The work is published in the Journal of The Minerals, Metals & Materials Society 71 (2019).

Zakaria (left) and Wenbo (right) explaining nanomaterials to high school students in the Singh Center for Nanotechnology, outside PI Pikul’s lab space.
Broader impacts:
Students from our research group participated in NanoDay 2018, which is an outreach event for local High School Students throughout the Philadelphia Region. This date, 10/9, pays homage to the nanometer scale, 10-9 meters.
Graduate students, Zhimin and Zakaria, described approaches for fabricating nanoporous materials with outstanding mechanical properties and promising energy storage applications. A Master’s student, Wenbo, demonstrated the self-assembly of two-dimensional materials, graphene and MXene. Groups of high school students that visited our table were especially fascinated by the natural coloration of nanoporous materials, which led to many interesting discussions.
